 |
|
 |
Carbon & Graphite |
|
|
 |
SiC (CVR) |
|
|
 |
SiC (CVD) |
|
|
|
|
|
 |
General Characteristics |
|
 |
STRONG
RESISTANCE TO HEAT
: CAN BE USED IN HIGH-TEMPERATURE
CONDITIONS |
|
Carbon
and graphite can be
used at temperatures
of up to 3,000˚C in
inert atmospheres,
and can
also be used up to
a maximum of 400˚C
in air. |
| Atmosphere |
Maximum
temperature
at which
these
can be
used |
Air
Steam
Hydrogen
Vacuum
Nitrogen
Chlorine
Argon
|
400
˚C
700 ˚C
1,000
˚C
2,200
˚C
2,200
˚C
2,500
˚C
3,000
˚C |
|
 |
THERMAL
EXPANSION IS SMALL
: DIMENSIONS THEREFORE
REMAIN STABLE |
|
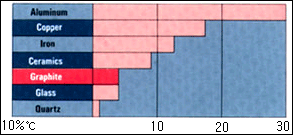
The
gragh at right exhibits a comparison
thermal
expansion coefficient of graphite
and other
materials. The thermal expansion
figures for
carbon and graphite are considerably
lower than those for metals.
|
 |
 |
CARBON
AND GRAPHITE ARE GOOD CONDUCTORS
OF HEAT |
The
gragh at right compares the thermal
conductivities of graphite and
other materials.
Graphite has high thermal conductivity.
|
 |
|
| |
 |
THERMAL
SHOCK RESISTANCE |
The
following general
formula shows thermal
shock
resistance. Carbon
has high thermal conductivity
and low coefficient
of thermal expansion,
with a small Young's
modulus. Carbon therefore
has superior resistance
to thermal shock.
|
| Thermal
Shock Resistance (cal/cm·sec)
= |
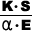 |
K
: Thermal Conductivity
(cal/cm·sec·˚C)
α : Confficient of
Thermal Expansion(1/˚C)
S : Thesile Strength
(kg/㎠)
E : Young's Modulus
(kg/㎟) |
|
| |
Young's
Modulus(kg/㎟) |
| Graphite |
500 ~
1,500 |
Glass
|
600 ~
750 |
| Iron |
9,000 |
| Copper |
11,000 |
| Tungsten |
35,000 |
|
|
 |
GRAPHITE'S
STRENGTH INCREASES AT HIGH TEMPERATURES |
|
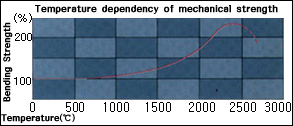
When
metallic materials are heated
to high
temperatures , their strengths
decrease, but
graphite becomes stronger at high
temperatures.
|
 |
|
|
| |
 |
GRAPHITE
IS STRONGLY RESISTANT TO CORROSION
AND IS NOT
ATTACKED BY CHEMICALS |
Graphite
is generally not corroded by acide
and alkalis at room temperatures.
Mixed acids,
such as a mixture of phosphoric
acid and potassium dichromate,
can oxidize graphite |
|
 |
GRAPHITE
IS LIGHT IN WEIGHT AND CAN BE
PROCESSED EASILY |
|
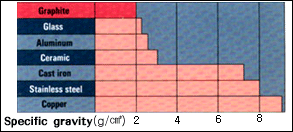
The
apparent density of graphite is
around 2, less
than that of aluminum, the lightest
of metals.
The specific gravity of graphite
is 1/3 of copper or
iron. Graphite is easily machined,
so that precise
machining of graphite can be avaiable.
|
 |
| |
 |
GRAPHITE
HAS INHERENT LUBRICITY |
| Graphite
has inherent lubricity, and will
not damage other materials with
which it comes in contact. |
 |
MOLTEN
METALS AND MOLTEN
GLASS DO NOT ADHERE
TO GRAPHITE |
|
|
|
|
 |
